| Clinical Effects of RP101 in Pancreatic Cancer |

|

|

|
|
The following clinical studies suggest that orally administered RP101 in combination with standard chemotherapy could represent a significant advance in the treatment of metastasized and locally advanced pancreatic cancer. First Clinical Study13 patients with metastatic and locally advanced pancreatic cancer (9 subjects with Stage IV and 4 subjects with Stage III) were treated with gemcitabine, cisplatin and RP101. Ten subjects (77%) survived more than 1 year and after one year there were close to four times more patients who had metastatic disease at the start of therapy alive in the RP101 group compared to the chemotherapy alone control group: 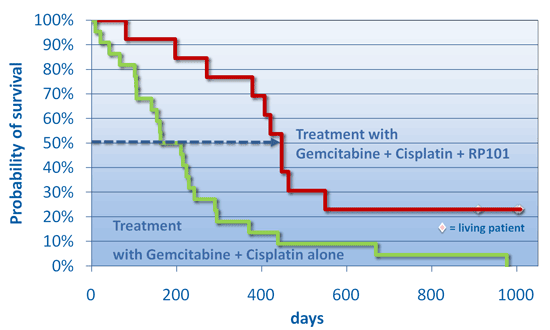
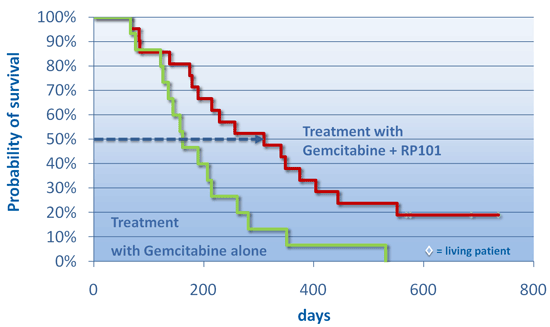
Second Clinical StudyA 22-patient dose ranging study of RP101 in the treatment of advanced pancreatic cancer was performed at 3 sites in Germany and the results were nearly identical to those from the first study. 8 subjects (38%) survived more than 1 year (control group only 9.5%) and after one year there were close to five times more patients who had metastatic disease at the start of therapy alive in the RP101 group compared to the chemotherapy alone control group:
|