8th Guide to German Biotech Companies 2006: German Biotechs 2006

|
RESprotect
Name
Address/P.O. Box
Postal Code/City State
Contact Person
Telephone
Fax
Email Address
Internet Website
Number of Employees
Founded (year)
Type of Laboratory
Areas of Activity
Annual Turnover
Relevant R&D Budget
Biological Patents
External Collaborations
Request for
Further Collaborations
|
RESprotect GmbH
Fiedlerstr. 34
D-01307 Dresden
Saxony
Prof. Dr. Rudolf Fahrig
+49-351-4503200
+49-351-4503210
This e-mail address is being protected from spam bots, you need JavaScript enabled to view it
www.resprotect.com
10
2000
S1
Cancer chemotherapy;
Chemotherapy of infectious diseases
n.a.
n.a.
n.a.
WITEGA/Berlin; Nycomed/Linz,
Austria; ERCOM/Budapest,
Hungary; Clinics Chemnitz;
University Leipzig; University
Munich; Technical University
Munich;University Vienna/
Austria; Avantogen/San Diego,
USA.
Partnering: RESprotect is looking for the appropriate partner to develop its key project RP101 in Europe, South America and Asia,
and its follow-on compounds worlwide.
|
RESprotect GmbH is a privately owned biotechnology company located in Dresden Germany. RESprotect is focusing on the inhibition of chemoresistance and the enhancement of chemosensitivity. In contrast to the well known efforts to circumvent or decrease existing chemoresistance, this basic approach is unrivalled. RESprotect was founded in 2000. The founder is geneticist and came from the Fraunhofer-Institute for Toxicology in Hannover. At present clinical studies with RP101 in the pancreas cancer indication, exploratory research in the identification of next generation of New Chemical Entities (NCEs) and general broadening of clinical indications are underway. RESprotect is in a position to enter exclusively a segment in the huge market of anticancer cytostatics. Use patents exist and extension of the patent portfolio by substance patents is achieved. New chemical entities have been identified and introduced to the development pipeline.
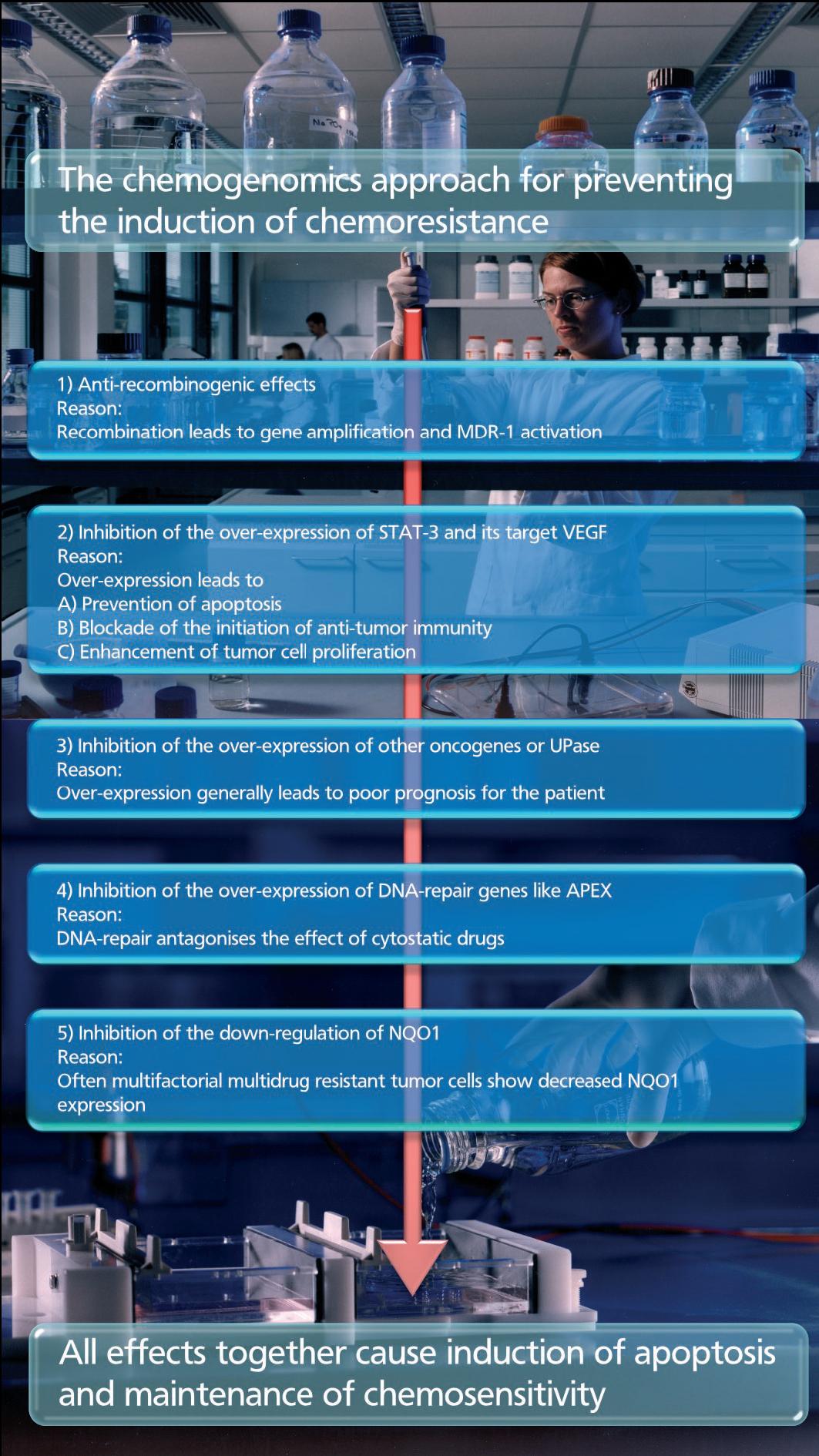
In cancer model systems, chemoresistance is often mediated by a single gene, and, therefore, may in theory be inhibited by any drug that targets the product of that gene. All these drugs possess potency and specificity exclusively for only one of the several reasons for chemoresistance. In this respect, the chemogenomics approach focuses on small molecules, causing favorable phenotypic changes, and inhibiting or preventing the induction of chemoresistance. The drugs have to counteract the over-expression of apoptosis-antagonizing genes and to enhance the immune responses. By influencing not only one but a number of different validated targets a new class of effective anti-cancer drugs will be developed. These compounds have to be administered in addition to standard chemotherapy. RP101 is the first drug that shows these effects in tumor cells in culture, in animals and in patients. RP101 improves the efficacy of chemotherapy in treating pancreatic carcinoma cells or patients. In pre-clinical studies, RP101 has shown strong antitumor effects due to inhibition of |
|
chemoresistance and the enforcement of apoptotic response upon cancer drug treatment. RP101 affects numerous gene products related to chemoresistance and tumor immunity. In a Phase I study including five different tumor entities and 12 different cytostatic drugs, no enhancement of unwanted side effects had been observed. In a Phase II pilot study with 13 pancreas cancer patients, RP101 co-treatment enhanced remissions, survival and time to progression. The results of the pilot study were confirmed in a second study with 21 patients in similar stages of disease. The results were not identical but similar. Our two studies roughly showed the tendency to double the survival time. In both studies, adverse events were consistent with those observed with the cytostatic drugs alone, or the underlying disease. The efficacy of RP101 exceeds all other regimens. The data implicated that acquisition of chemoresistance was prevented and the antitumor efficacy of standard chemotherapy was improved. A Phase II/III study with larger number of patients is in progress.
CEO – Founder:
Prof. Dr. Rudolf Fahrig
CSO – Cell and molecular biology:
Dr. Jörg-Christian Heinrich
CSO – Pharmacy and chemistry:
Dr. Dieter Lohmann
Finances:
Kerstin Jahn
Nearly 7 million euros were raised by the company in the first round of financing. The next round was replaced by out-licensing the North-America rights of RP101. RESprotect has signed in September 2004 a license agreement with Australian Cancer Technology. The Australian biotech company acquired the license for the use of the anti-cancer drug RP101 in North America. The drug is the first commercial breakthrough of RESprotect. AustCancer (new name Avantogen) will finance the clinical development of RP101 in Germany and the USA. RESprotect or its licensee will have free access to the data for approval of RP101 in Europe or elsewhere outside the USA and Canada. |
RESprotect
|