Prevention of adriamycin-induced mdrl gene amplification and expression in mouse leukemia cells by simultaneous treatment with the anti-recombinogen bromovinyldeoxyuridine
Authors: Fahrig R.; Steinkamp-Zucht A.; Schaefer A.1
RESprotect GmbH - Prevention of Chemoresistance, Fiedlerstraße 34, D-01307 Dresden and 1Fraunhofer Arbeitsgruppe für Toxikologie und Umweltmedizin, Hamburg/Germany
Summary
The anti-recombinogenic substance (E)-5-(2-bromovinyl)-2 -deoxyuridine (BVDU) was tested for its ability to prevent adriamycin-induced mdrl gene amplification and expression in mouse leukemia cells in vitro.
F4-6 cells that were treated with stepwise enhanced doses of adriamycin
acquired resistance against adriamycin. While 20 ng/ml adriamycin
showed strong toxic effects in sensitive cells, the same dose was
tolerated at the end of the long-term experiment following treatment
with stepwise enhanced doses of adriamycin. In parallel experiments,
0.5 or 1
-deoxyuridine (BVDU) was tested for its ability to prevent adriamycin-induced mdrl gene amplification and expression in mouse leukemia cells in vitro.
F4-6 cells that were treated with stepwise enhanced doses of adriamycin
acquired resistance against adriamycin. While 20 ng/ml adriamycin
showed strong toxic effects in sensitive cells, the same dose was
tolerated at the end of the long-term experiment following treatment
with stepwise enhanced doses of adriamycin. In parallel experiments,
0.5 or 1  g/ml
BVDU was given together with adriamycin. BVDU prevented the formation
of resistance against adriamycin treatment. Using differential PCR, the
signal intensity of the mdrla-specific band appeared markedly
increased in adriamycin-resistant cells, while the signal intensities
of the adriamycin + BVDU-treated cells resembled the intensity ratio of
the untreated control cells. Beyond that, in resistant F4-6 cells
increased expression of mdr genes was demonstrated by Northern blot analysis.
g/ml
BVDU was given together with adriamycin. BVDU prevented the formation
of resistance against adriamycin treatment. Using differential PCR, the
signal intensity of the mdrla-specific band appeared markedly
increased in adriamycin-resistant cells, while the signal intensities
of the adriamycin + BVDU-treated cells resembled the intensity ratio of
the untreated control cells. Beyond that, in resistant F4-6 cells
increased expression of mdr genes was demonstrated by Northern blot analysis.
Key words
gene amplification/leukemia/mouse cells/multidrug resistance/recombination
Development of multidrug resistance (MDR) in hamster, mouse and human cell lines is frequently associated with mdr-gene amplification, and the level of drug resistance is often correlated with the level of gene amplification (Gottesman, 1993). mdr-Gene amplification in association with multidrug resistance in mice and rats has been observed not only in vitro, but also in vivo. Mouse leukemia cells, selected in vivo for resistance to anthracyclines, have developed mdr-gene amplification (Demidova et al., 1987; Gudkov et al., 1991; Volm et al., 1991; Schimke et al., 1986).
As it is generally agreed that the first steps of gene amplification and recombination are the same (Schimke et al., 1986; Volkert & Broach, 1986; Wahl, 1989), both processes may be causally related. To test this hypothesis, in previous experiments co-recombinogens and anti-recombinogens were studied for their ability to enhance or suppress the cytostatica induced formation of SV40 amplification. There could be observed a very strong correlation between both effects (Fahrig & Steinkamp-Zucht, 1996). (E)-5-(2-bromovinyl)-2´-deoxyuridine (BVDU) was the only substance within the group of anti-recombinogens with likely clinical significance (Fahrig, 1996). Therefore, this substance was selected for the present in vitro-experiments using a mouse tumor cell line that is known to develop adriamycin resistance corresponding to mdr-gene amplification. F4-6 tumor cells are Friend virus transformed mouse erythroleukemia cells (Ostertag et al., 1972). Cell line F4-6wt shows the wild type, i.e., is drug sensitive and has no increased mdr-gene expression and amplification.
F4-6 cells were treated with stepwise enhanced doses of adriamycin. In the course of these experiments the cell population acquired resistance against adriamycin. While 20 ng/ml adriamycin showed strong toxic effects in sensitive cells, the same dose was tolerated at the end of the long-term experiment. In parallel experiments, adriamycin was given together with 0.5 or 1 µg/ml BVDU (Fig. 1 A). At these doses, BVDU had no toxic effects when given alone (Fig. 1 B). It is apparent that BVDU prevented the formation of resistance against adriamycin treatment. The tumor cells remained sensitive against adriamycin treatment.
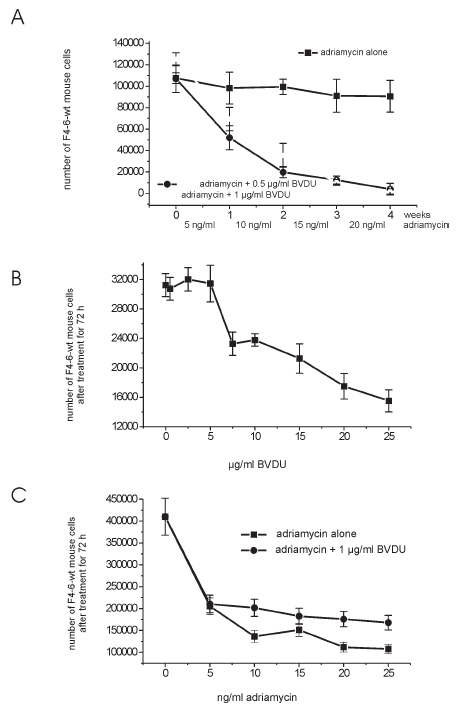 Figure 1:
Figure 1:
(A) Treatment of mouse erythroleukemia F4-6-wt cells (drug sensitive, no expression of mdr genes) for 4 weeks with stepwise enhanced doses of adriamycin, or adriamycin + BVDU.
(B) Treatment of mouse erythroleukemia F4-6-wt cells (drug sensitive, no expression of mdr genes) for 72 h with different doses of BVDU.
(C) Treatment of mouse erythroleukemia F4-6-wt cells (drug sensitive, no expression of mdr genes) for 72 h with different doses of adriamycin, or adriamycin + 1
μg/ml BVDU.
In particular, 105 F4-6 cells were seeded into 5 ml DMEM (Dulbeccos´s Minimum Essential Medium). Four days (86 h) later, the medium was replaced by fresh medium, and adriamycin alone, BVDU alone or BVDU + adriamycin were added to the cultures. After 72 h treatment time the medium was replaced by fresh medium and the same or higher concentrations of the substances were added. The toxic effect of adriamycin was so strong that the treatment had to be interrupted from time to time for four days where the cells were allowed to grow without treatment. In contrast to this, the growth curves for BVDU (0.5 and 1 µg/ml) were similar to those of the untreated control. Therefore, the cells of these cultures had to be serially passaged.
To determine the number of living cells, a CASY 1 cell counter and analyser system (Winkelmeier et al., 1993) was used. Cell counting and volume determination is based on the displacement of conductive electrolyte by dielectric cells. The signals generated by the cells suspended in an electrolyte are evaluated by pulse area analysis. The pulse area of the signal is strictly proportional to the volume of the particle generating the signal. In dead cells the integrity of the cell membrane is lost. This loss increases the conductivity and reduces the pulse area of the electric signal. Thus, to exclude debris and dead cells, only particles with a size of 6 – 10 µm were counted as vital cells.
To ensure that mdr gene amplification is involved during the treatment of F4-6 cells, we performed a differential polymerase chain reaction as described by Frye et al. (Frye et al., 1989). The method is based on a simultaneous PCR amplification of a single copy gene together with the gene of interest (here mdr1a). It has been shown that the PCR product yield (i.e. differences in signal intensities) is preserved during later cycles with differential PCR but not with conventional PCR.
Interferon- g (IFNG) was used as single copy reference gene. The DNA from appr. 1 x 106 cells was purified with the „InViSorb“ genomic DNA purification kit (InViTek, Berlin, Germany). The PCR mixtures (reaction volume 50µl) contained the target DNA sample, 200 pM of each primer, 1 Unit Taq polymerase, 0.25 mM of each dNTP and a reaction buffer containing 50 mM KCl, 1.5 mM MgCl2, 10 mM Tris-HCl (pH 8.3). Aliquots of the genomic DNA were amplified as follows: Initial denaturation at 99° C for 3 minutes, initial annealing with addition of Taq polymerase at 52° C for 4 min. The following 35 cycles included 30 sec of denaturation and annealing (96° C, 52° C) and an extension at 72° C for 2.5 min except for the final extension step which had 12.5 min. PCR reactions were performed using 4 different primers: MMIFN1:5’-GTGCTGCCGTCATTTTCTGC-3’, MMIFN2: 5’-CCCTTTCTTCCCTTC TTCGTTC-3’, MMMDR1A-3: 5’-GGTGGATATGGGGGACTTTTGG TA-3’, MMDR1A-4: 5’-CCCCGTGCAGGCTTTTCTTCA-3’.
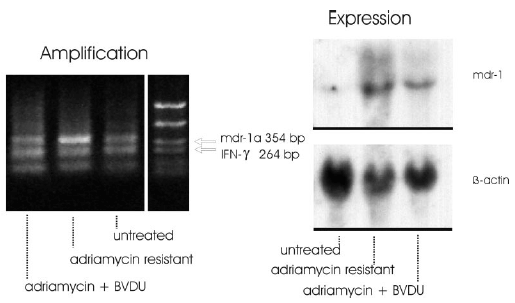
Figure 2:
(Right) Detection of mdr gene expression by Northern blot analysis of RNA: F4-6-wt (wild-type) cells. F4-6 cells, resistant to adriamycin after treatment with stepwise enhanced doses of adriamycin. F4-6-wt cells, not resistant to adriamycin after treatment with stepwise enhanced doses of adriamycin in combination with BVDU.
(Left) Detection of amplified mdr1a gene by differential PCR: F4-6-wt (wild-type) cells; F4-6 adriamycin-resistant cells; and F4-6-wt cells treated for 4 weeks with stepwise enhanced doses of adriamycin up to a dose of 20 ng/ml in combination with 1 μg/ml BVDU.
As result of these experiments it can be stated that the signal intensity of the mdr1a specific band appeared markedly increased in adriamycin resistant cells. The signal intensities of the adriamycin + BVDU treated cells resembled the intensity ratio of the untreated control cells (Fig. 2 left).
The expression of the mdr1-gene was studied in adriamycin-sensitive and in adriamycin-resistant F4-6 cells selected using Northern blot analysis. Levels of b-actin mRNA were also analyzed comparatively. b-Actin was used as an internal control for equivalent amounts of RNA loaded. Total cellular RNA from drug-sensitive and -resistant F4-6 cells was isolated by the method of Chomczynski & Sacchi (1987). 30 µg RNA was denatured by glyoxalation, electrophoresed through a 1% agarose gel, and transferred to Biodine A membrane (PALL, Portsmouth, UK) by the capillary blotting technique using 20 x SSD solution (1 x SSD contains 0.15 M sodium chloride and 0.015 M sodium citrate). RNA was immobilized by baking the membrane at 80° C for 1 h. DNA probes for hybridization were labeled with [a-32P]-dCTP by the random primer method using the Multiprime DNA labeling system from Amersham (Braunschweig, FRG). Membrane hybridization was carried out after 6 h prehybridization as described by Braun et al. (1989). Hybridized blots were washed in 2x SSD and 0.1 % SDS at room temperature for 20 min and at 50° C for 60 min, in 0.5 x SSC and 0.1 % SDS at 50° C, and finally in 0.2 x SSC and 0.1 % SDS at 55° C for 30 min. The membranes were exposed at –80° C to an X-ray film (Fuji Photo Film, Tokyo/Japan) using intensifying screens. The membranes were sequentially hybridized with the DNA probes. The following probes were used for hybridization: human mdr1 probe pHDR5A, 1.38 kb, and human b-actin cDNA probe, 1.2 kb.
Table I
Comparison of IC50 and the corresponding concentrations (n = 3 +/- SE)
| Untreated control |
Adriamycin (ng/ml) |
BVDU (μg/ml) | Adriamycin + BVDU |
|
|
F4-6-wt (wild-type) cells |
||||
| IC50 | - | 5 | 25 | 10 + 1 |
| No. of viable cells | 410 000 +/- 39 250 31 250 +/- 1562 |
205 250 +/- 19 125 - |
- 15525 +/- 1500 |
201 750 +/- 19 375 - |
|
F4-6 cells, resistant to 20 ng/ml adriamycin after treatment with stepwise anhanced doses of adriamycin |
||||
| IC50 | - | 35 | - | 35 + 1 |
| No. of viable cells | 305 750 +/- 29 250 |
140 500 +/- 13 900 |
- | 160 250 +/- 14500 |
In resistant F4-6 cells increased expression of mdr-genes (Fig. 2 right), and in wild-type adriamycin-sensitive F4-6 cells mdr transcripts at scarcely detectable levels could be clearly demonstrated. This position corresponds to the size of the mdr1b mRNA (Hsu et al., 1989). In comparison, in the adriamycin-resistant cells, transcripts ranging from 4.5 to 6 kb reacted very strongly with the mdr1 cDNA probe. As discussed previously (Schaefer et al., 1993), the combined message obviously results from the crossreaction of the human mdr1 cDNA probe with the mouse mdr1a and mdr1b mRNAs and from the heterogeneity in the size of mdr1a transcripts (Hsu et al., 1989; 1990).
It is apparent that BVDU + adriamycin treatment led to mdr transcripts at more than five times lower levels than treatment with adriamycin alone, and that BVDU inhibited the formation of mdr-gene amplification. In reality the preventing effect of BVDU was much higher than is expressed in the lower levels of mdr transcripts because the cells that did not undergo mdr-gene amplification were killed during treatment and could not be detected. At the end of treatment only cells survived that were resistant at least to a certain extent. Therefore, the more relevant effect is the inactivation of cells seen in Fig. 1 A.
The effectiveness of DNA replication or repair of DNA damage depends on a balanced supply of deoxyribonucleoside triphosphate (dNTP) precursors of DNA. Should cellular dNTP levels be perturbed, a wide range of genetic events may follow due to aberrant DNA replication or repair. For example, manifestations of DNA precursor imbalance can include gene mutation; recombination; enhanced sensitivity to mutagens/carcinogens (i.e. co-mutagenic and co-carcinogenic effects); chromosome breakage, exchange or loss (Haynes, 1985; Kunz et al., 1994). Therefore, thinking about the mode of action of 5-substituted pyrimidine nucleoside analogs, possible changes in nucleotide pools have to be taken into account. It seems evident that for the repair of cytostatica induced DNA-damage this pool could be very important, and that the addition of 5-substituted pyrimidine nucleoside analogs could cause modifications not only in the available pools but also by changing the fidelity of certain polymerases. Such changes could influence recombination as well as gene amplification.
Theoretically it is possible that BVDU, by disrupting nucleotide pools or by inhibiting key enzymes, might act as general inhibitor of DNA repair. As such, inhibition of recombination may be only one outcome. BVDU may also act to increase the cytotoxicity of chemotherapeutic agents by blocking repair of induced DNA lesions.
To demonstrate that BVDU does not simply act as a general inhibitor of DNA repair just increasing the cytotoxicity of adriamycin by blocking repair of adriamycin induced DNA lesions, F4-6-wt sensitive cells were treated for 72 hours with up to 25 ng/ml adriamycin alone or with adriamycin + 1 µg/ml BVDU. It can be seen in Fig. 1 C and table 1 in respect to the IC50 that BVDU did not enhance the effect of adriamycin. In contrary, the toxic effect of adriamycin seems to be lowered. This reduction of toxicity could also be observed in vivo: tumor bearing rats treated with adriamycin alone showed at longer treatment times a strong loss of body weight. Rats treated with adriamycin (3 x 4 mg/kg per week) + BVDU (5 x 15 mg/kg per week) showed an increase in body weight and decrease in tumor weight (unpublished results).
Drugs that reverse MDR have been shown to inhibit the P-glycoprotein-mediated drug efflux mechanism (Ford & Hait, 1990). Therefore, another question was, if it is perhaps possible to circumvent multidrug resistance by treatment of resistant tumor cells (overexpressing the mdr-gene) with BVDU. F4-6 cells (resistant to 20 ng/ml adriamycin), collected at the end of the 4 week experiment, were treated for 72 hours with 35 ng/ml adriamycin alone, or with adriamycin + 1 µg/ml BVDU. It is apparent (table 1) that BVDU did not influence the effect of adriamycin. The IC50 was similar for both treatments.
Although not directly proven, virtually all proposed mechanisms for gene amplification involve recombination (Schimke et al., 1986; Volkert & Broach, 1986; Wahl, 1989). In fact, it is difficult to think of any model for gene amplification that would not involve recombination. Therefore, it was of interest to see if the anti-recombinogenic effect of BVDU in yeast (Fahrig, 1996) that had been confirmed by the anti-amplification effect on SV40 DNA in Chinese hamster cells (Fahrig & Steinkamp-Zucht, 1996) could be observed also in a relevant mammalian gene. As this was the result of our study, the idea is supported that gene amplification is mechanistically related to recombination. In contrast to those drugs used for reducing the function of P-glycoprotein, the action of BVDU is directed against the mechanism of resistance, i.e., amplification of several target genes may be attacked by which many neoplastic cells develop resistance to anticancer drugs. We have shown that mdr-gene amplification and expression could be detected in adriamycin treated and resistant cells in vitro, underlining the hypothesis that gene amplification is part of the mechanism how these cells gain resistance against antineoplastic drugs.
Chronic treatment with antineoplasic drugs does not only lead to amplification of the mdr-gene, but also to the overexpression and amplification of certain oncogenes controlling resistance to this treatment. The results of Bhushan et al. (Bhushan et al., 1992) are consistent with the hypothesis that induction of c-fos by low levels of cytotoxic drugs may be an early event in the acquisition of the MDR phenotype. Furthermore, Bhuschan et al. (1996) could show that enhanced expression of the c-fos gene preceded the expression of the mdr3 gene. In MDR sublines of both murine sarcoma and human KB-3-1 carcinoma cell lines, the expression of c-fos and c-jun increased as drug resistance increased. Nakagawara et al. (1990) found an inverse correlation between expression of amplified N-myc and the expression of mdr1 in the same human neuroblastoma tumor samples. Neuroblastoma with N-myc amplification no longer responded to chemotherapy. Bordow et al. (1994) and Norris et al. (1996) observed that expression of the multidrug resistance-associated membrane-bound glycoprotein (MRP) gene correlated with amplification and overexpression of the N-myc oncogene in childhood neuroblastoma, which is central to the malignant phenotype of this disease. Therefore, gene amplification may be a central mechanism in acquiring chemoresistance.
Our in vitro study with adriamycin + BVDU has been reconfirmed in vivo in our laboratory and in an industrial laboratory. BVDU inhibited at plasma levels that were similar to those in the in vitro-experiments adriamycin-induced amplification and expression of the mdr-1 gene in tumors of rats in vivo. Through this inhibition of gene amplification, BVDU strongly enhanced the effects of adriamycin in suppressing the growth of fibrosarcomas, mammary adenocarcinomas and Ah13 sarcomas. At present we examine the effects of BVDU in vivo in combination with different cytostatic drugs. Resistance against these drugs has to do with amplification/expression of other genes than the mdr1 gene.
Acknowledgements
We thank Dr. H.-D. Zucht (Niedersächsisches Institut für Peptid-Forschung, Hannover/Germany) for his help in measuring gene amplification. (E)-5-(2-bromovinyl)-2´-deoxyuridine (BVDU) was a gift from Berlin-Chemie/Berlin/Germany. Friend mouse erythroleukemia cells, line F4-6 were kindly provided by Prof. W. Ostertag (Heinrich Pette Institute/Hamburg/Germany). The mdr1 probe pHDR5A was generously provided by Dr. M. M. Gottesman, National Cancer Institute, NIH, Bethesda, Md., USA; the human b-actin cDNA probe was kindly provided by Dr. T. Braun, Institute of Biochemistry and Biotechnology, University of Braunschweig, Germany).
References
Bhushan,A., Abramson,R., Chiu,J.F. & Tritton,T.R. (1992) Expression of c-fos in human and murine multidrug-resistant cells. Mol. Pharmacol., 42, 69-74.
Bhushan,A., Slapak,C.A., Levy,S.B. & Tritton,T.R. (1996) Expression of c-fos precedes MDR3 in vincristine and adriamycin selected multidrug resitant murine erythroleukemia cells. Biochem. Biophys. Res. Commun., 226, 819-822.
Bordow,S.B., Haber,M., Madafiglio,J., Cheung,B., Marshall,G.M. &
Norris, M.D. (1994)
Expression of the multidrug resistance-associated protein (MRP) gene correlates
with amplification and overexpression of the N-myc oncogene in childhood
neuroblastoma. Cancer Res., 54, 5036-5040.
Braun,T., Bober,E., Buschhausen-Denker,G., Kotz,S., Grzeschik,K.-H.
& Arnold, H.H. (1989) Differential expression of
miogenic determination genes in muscle cells: possible autoactivation by the
myf gene products. EMBO J., 8, 3617-3625.
Chomczynski,P. & Sacchi,N. (1987) Single-step method of DNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem., 162, 156-159.
Demidova,N.S., Goncharova,S.A., Chernova,O.B., Kopnin,B.P. & Gudkov,A.V. (1987) Gene amplification in murine leukemia cells with multiple drug resistance acquired in vivo. Genetika, 23, 1797-1806.
Fahrig,R. (1996) Anti-recombinogenic and convertible co-mutagenic effects of (E)-5-(2-bromovinyl)-2’-deoxyuridine (BVDU) and other 5-substituted pyrimidine nucleoside analogs in S. cerevisiae MP1, Mutation Res., 372, 133-319.
Fahrig,R. & Steinkamp-Zucht,A. 1996) Induction or suppression of SV40 amplification by genotoxic carcinogens, non-genotoxic carcinogens or tumor promoters, Mutation Res., 356, 217-224.
Ford,J.M. & Hait,W.N.(1990) Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacol. Rev., 42, 155-199.
Frye,R.A., Benz,C.C. & Edison,L. (1989) Detection of amplified oncogenes by differential polymerase chain reaction. Oncogene, 4, 1153-1157.
GottesmannM.M. (1993) How cancer cells evade chemotherapy: sixteenth Richard and Hinda Rosenthal foundation award. Cancer Res., 53, 747-754.
Gudkov,A.V., Chernova,O.B. & Kopnin,B.P.(1991) Karyotype and amplicon evolution during stepwise development of multidrug resistance in Djungarian hamster cell lines, in: I. B. Roninson (ed.), Molecular and cellular biology of multidrug resistance in tumor cells, Plenum, New York; p. 147-168.
Haynes,R.H. (1985) Molecular mechanisms in genetic stability and change: The role of deoxyribonucleotide pool balance, in: F.J. de Serres (Ed.), Genetic Consequences of Nucleotide Pool Imbalance, Plenum, New York. p. 1-23.
Hsu,S.I.-H., Lothstein,L. & Horwitz,S.B. (1989) Differential overexpression of the mdr genes family members in multidrug-resistant J 774.2 mouse cells. Evidence that distinct P-glycoprotein precursors are encoded by unique mdr genes. J. Biol. Chem., 264, 12053-12062.
Hsu,S.I.-H., Cohen,D., Kirschner,L.S., Lothstein,L., Hartstein,M. & Horwitz,S.B. (1990) Structural analysis of the mouse mdr1a (P- glycoprotein) promoter reveals the basis of differential transcript heterogeneity in multidrug-resistant J 774.2 cells. Mol. Cell Biol., 10, 3596-3606.
Kunz,B.A., Kohalmi,S.E., Kunkel,T.A., Mathews,C.K., McIntosh,E.M. &
Reidy,J.A. (1994) Deoxyribonucleoside
triphosphate levels: A critical factor in the maintenance of genetic stability.
Mutation Res., 318, 1-64.
Nakagawara,A., Kadomatsu,K., Shin-ichi,S., Kohno,K., Akazawa,.K, Nose,Y.
& Kuwano,M.(1990) Inverse
correlation between expression of multidrug resistance gene and N-myc oncogene in human neuroblastomas. Cancer Res., 50, 3043-3047.
Norris,M.D., Bordow,S.B., Marshall,G.M., Haber,P.S., Cohn,S.L. & Haber,M.(1996) Expression of the gene for multidrug-resistance-associated protein and outcome in patients with neuroblastoma. N. Engl. J. Med., 334, 231-238.
Ostertag,W., von Ehrenstein,G. & Charache,S. (1972) Duplicated-chain genes in Hopkins - 2 haemoglobin of man and evidence for unequal crossing over between them. Nature New Biol., 237, 90-94.
Schaefer,A., Westendorf,J., Lingelbach,K., Schmidt,C.A., Mihalache,D.-L., Reymann, A. & Marquardt,H. (1993) Decreased resistance to N,N-dimethylated anthracyclines in multidrug-resistant Friend erythroleukemia cells. Cancer Chemoter. Pharmacol., 31, 301-307.
Schimke,R.T., Sherwood,S.W., Hill,A.B. & Johnston,R.N. (1986) Overreplication and recombination of DNA in higher eukaryotes: potential consequences and biological implications. Proc. Natl. Acad. Sci., USA, 83, 2157-2161.
Volkert,J.R. (1986) Site-specific recombination promotes plasmid amplification in yeast.Cell, 46, 541-550.
Volm,M., Mattern,J. & Pommerenke,E.W. (1991) Time course
of mdr gene amplification during in vivo selection for
adriamycin-resistance and during reversal in murine leukemia L 1210. Anticancer Res., 11, 579-586.
Wahl,G.M. (1989) The importance of circular DNA in mammalian gene amplification. Cancer Res., 49, 1333-1340.
Winkelmeier,P., Glauner,B. & Lindl,T. (1993) Quantification of cytotoxicity by cell volume and cell proliferation. ALTERNATIVES TO LABORATORY ANIMALS: ATLA, 21, 269.