Combating Chemoresistance –
Chemogenomics Joins the Battleground
![]()

Drugs that prevent or inhibit induction of chemoresistance are beset
by limitations. Rudolf Fahrig at RESprotect GmbH investigates a new approach
Rudolf Fahrig is Professor at the University of Hamburg, Germany, and CEO of RESprotect GmbH. He served for many years in several national and international scientific committees and on the editorial boards of scientific journals. His work, resulting in a drug that prevents induction of chemoresistance, received the ‘Central German Innovation Prize’ in 2005. Rudolf studied Biology at the University of Hamburg, the Free University, Berlin, the University of Vienna, Austria and the Justus-Liebig-University.
In cancer model systems, chemoresistance is often mediated by a single gene, and therefore may in theory be inhibited by any drug that targets the product of that gene. All these drugs possess potency and specificity for only one of the several reasons for chemoresistance. In this respect, the chemogenomics approach focuses on small molecules, causing favourable phenotypic changes, and inhibiting or preventing the induction of chemoresistance. The drugs have to counteract the over-expression of apoptosis-antagonising genes and to enhance immune responses. By influencing not only one but a number of different validated targets, a new class of effective anticancer drugs will be developed. These compounds have to be given in addition to standard chemotherapy. RP101 (BVDU) is the first drug that shows these effects in vitro and significantly extended patient survival rate in a clinical pilot study. Similar tendencies are observed in a second study that has started recently.
Cytotoxic anticancer drugs are used in chemotherapy to destroy proliferating tumour cells. Unfortunately, repeated chemotherapeutic treatment induces or selects for chemoresistance of remaining cancer cell populations. Chemoresistant cancer cells are often characterised by altered gene expression and genomic instability because of mutation, recombination and gene amplification events. Deregulation of DNA-repair enzymes is part of this phenomenon (for example, p53 gene, BRCA1/2, UBE2N, APEX and Rad51). Furthermore, the activity of enzymes that metabolise and bioactivate drugs (for example, DHFR and NQO1) or proteins that transport cytotoxic agents (for example, gp120) is often abnormal in chemoresistant cells. Therefore, the success of cytotoxic drug treatment, which aims to eliminate tumour burden, is reduced by cellular resistance.
Although manifestations of resistance are conventionally referred to as acquired or intrinsic on the basis of the initial response to the first therapy, a common feature is the development of a phenotype resistant to a variety of structurally and functionally distinct agents. In both manifestations, drug resistance is a multifactorial phenomenon involving multiple interrelated or independent mechanisms (1).
In model systems, resistance is often caused by the overproduction of glycoprotein gp120, which is coded by the multi-drug resistance gene mdr1, and, in theory, targeting this single mechanism should suffice to reverse chemoresistance. Several inhibitors of gp120 have already been tested in the clinic. However first generation agents (for example, cyclosporin, verapamil) were limited due to unacceptable toxicity, whereas second generation agents (for example, valspodar, biricodar) had better tolerability but were restricted by unpredictable pharmacokinetic interactions and interactions with other transporter proteins. Third generation inhibitors (tariquidar XR9576, zosuquidar LY335979, laniquidar R101933 and ONT-093) have high potency and specificity for P-gp. Nevertheless, most resistant tumours use more than one mechanism to elude the therapy. Therefore, the use of drugs inhibiting gp120 is restricted to only a few tumours.
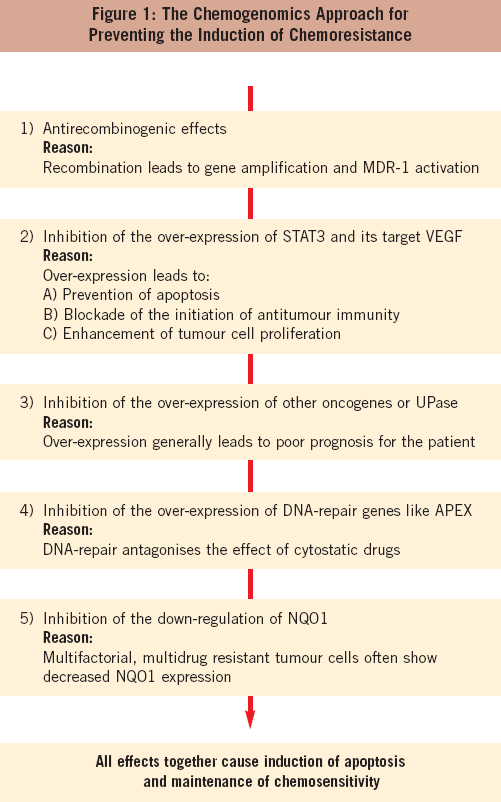 Figure 1 shows selected genetic effects that contribute to chemoresistance. These effects can be stratified as follows:
Figure 1 shows selected genetic effects that contribute to chemoresistance. These effects can be stratified as follows:
Level 1: The target of this level is increased recombination as: (a) a mechanism of the amplification of resistance and oncogenes, and (b of the activation of the MDR-1 gene. Mickley et al (3) demonstrated that chromosomal rearrangements with overexpression of hybrid MDR-1 mRNAs are a mechanism of acquired drug resistance. Antirecombinogenic drugs belong to different chemical classes, for example phenols, phorbol esters, base analogues and hormones.
Level 2: STAT3 promotes cell survival and renders cancer cells resistant to chemotherapy. Conversely, inhibition of STAT3- signalling increases sensitivity to chemotherapeutic agents (4). Downregulation of STAT3 and its target VEGF probably leads to an increase of antitumour immunity that is reflected by the enhanced expression of LTA and LTB, tumour necrosis factor LIGHT/TNFSF-14, and natural killer cell transcript 4 (NK4, IL-32).
Level 3: This involves inhibition of genes related to poor prognosis like the oncogene JUN-D or uridine phosphorylase (UPase). UPase gene expression seems to be strictly controlled by oncogenes, tumour suppressor genes and cytokines, and its activity is usually elevated in various tumour tissues. UPase is a marker of poor prognosis in several carcinoma (5).
Level 4: Several genes affecting chemoresistance are linked to DNA repair, for example UBE2N and APEX. The protein complex containing UBE2N seems to be involved in the assembly of novel polyubiquitin chains for signalling in DNA repair and, through differential ubiquination of PCNA, affects resistance to DNA damage. Silencing of APEX expression by RNA interference nearly doubled the specific lysis of cells with enhanced DNA nicking (6). Inhibition of DNA repair genes like APEX1 during anticancer treatment increases chemosensitivity.
Level 5: In accordance with the observation that a multifactorial multidrug resistance phenotype of tumour cells involves a decreased NQO1 expression (7), it should be beneficial for chemoresistance inhibitors to induce NQO1.
Inhibitors for receptors of the tyrosine kinase pathway, such as ERBB2 and inhibitors of the expression of single oncogenes like STAT3, are evolving too but have similar limitations.
Antiapoptotic survival pathways involve oncogenes like STAT3 and JUN-D (2) Constitutively activated STAT3 is oncogenic and contributes to the development of various human cancers by inhibiting apoptosis. Thus, STAT3 promotes cell survival and renders cancer cells resistant to chemotherapy. Accordingly, the inhibition of STAT3 signalling induces apoptosis specifically in tumour cells, and increases sensitivity to chemotherapeutic agents. Hence, the management of chemoresistance, at its best, has to address all of the options a cell is capable of to render therapy ineffective.
Several issues need to be addressed in order to develop drugs that inhibit or prevent induction of chemoresistance. The effectivity of drugs on the molecular level is one matter; their effectiveness in vitro and in vivo another. In the long run, in vitro treatment of tumour cells with cytotoxic drugs leads to the induction of resistance and the increase of cells. Co-treatment with a chemoresistance inhibitor maintains the chemosensitivity of cells. Drugs that are not effective with respect to maintenance of chemosensitivity are excluded from further studies. On the next level, the candidates are tested in in vivo tumour models. Naked mouse experiments are not advisable because the prediction of effectiveness is not better than that of in vitro experiments. Candidates who pass this barrier are tested for their toxicological profile. Cytotoxic substances are most probably detected before the in vitro and in vivo experiments and have been already excluded. Only promising candidates reach the toxicology level. The most promising candidate is tested in clinical trials. To my knowledge, none of the drugs that are in the developmental pipelines of pharmaceutical and biotech companies throughout the world are intended to inhibit or prevent induction of chemoresistance. Drugs which circumvent or attack already existing chemoresistance address only gp-120 (MDR-1) with varying specificity, but gp-120 is only one of several reasons for chemoresistance. Inhibitors of receptors for the tyrosine kinase pathway such as ERBB2 (Herceptin) and inhibitors of the expression of single oncogens or STAT3 are in development too. However, the use of such drugs is restricted to tumours with a specific genetic profile.
Chemoresistance is a multifactorial event, but since the development of inhibitors of chemoresistance is focused only on one target mechanism, no valuable drugs exist so far. There are several substances that are active on one or two levels. Besides the compounds mentioned previously, base analogs like (E)-5-(2-bromovinyl)-2’-deoxyuridine (BVDU, RP101); 5-iodo-2’-deoxyuridine (IDU), 5-iodo-2’-deoxycytidine (IDC); 2´-deoxy-5-trifluoromethyl-uridine (DTFMU), are active as antirecombinogens. However, since many base analogs are cytostatic, they do not reach the higher levels of testing.
The first drug showing the desired profile is BVDU (RP101). In a clinical pilot study with 13 pancreas cancer patients, survival and time to progression was roughly doubled. The same tendency was seen in a follow-up study that started in December 2004.
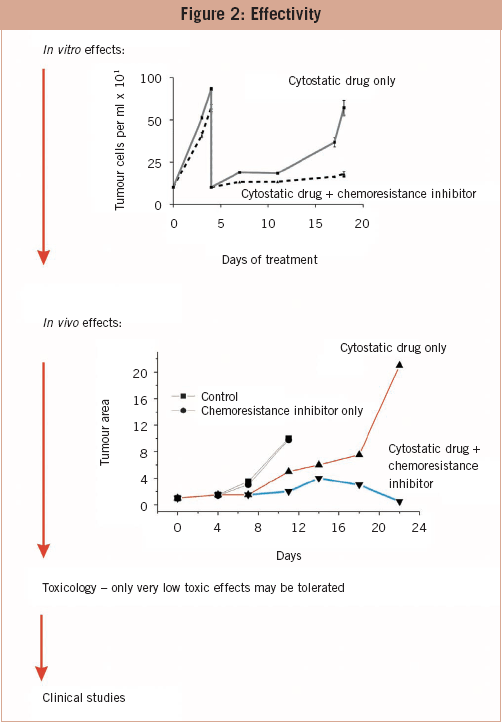
The effectivity of drugs on the molecular level is one matter; their effectiveness in vitro and in vivo another. In the long run, in vitro treatment of tumour cells with cytotoxic drugs leads to the induction of resistance and the increase of cells. Co-treatment with a chemoresistance inhibitor maintains the chemosensitivity of cells. Drugs that are not effective with respect to maintenance of chemosensitivity are excluded from further studies. On the next level, the candidates are tested in in vivo tumour models. Naked mouse experiments are not advisable because the prediction of effectiveness is not better than that of in vitro experiments. Candidates who pass this barrier are tested for their toxicological profile. Cytotoxic substances are most probably detected before the in vitro and in vivo experiments and have been already excluded. Only promising candidates reach the toxicology level. The most promising candidate is tested in clinical trials. To my knowledge, none of the drugs that are in the developmental pipelines of pharmaceutical and biotech companies throughout the world are intended to inhibit or prevent induction of chemoresistance. Drugs which circumvent or attack already existing chemoresistance address only gp-120 (MDR-1) with varying specificity, but gp-120 is only one of several reasons for chemoresistance. Inhibitors of receptors for the tyrosine kinase pathway such as ERBB2 (Herceptin) and inhibitors of the expression of single oncogens or STAT3 are in development too. However, the use of such drugs is restricted to tumours with a specific genetic profile.
References
- Gatti L and Zunino F, Overview of tumour cell chemoresistance mechanisms, Methods Mol Med 111: pp127-148, 2005
- Fahrig R, Heinrich JC, Nickel B, Wilfert F, Leisser C, Krupitza G, Praha C, Sonntag D, Fiedler B, Scherthan H and Ernst H, Inhibition of induced chemoresistance by cotreatment with (E)-5-(2-bromovinyl)-2’-deoxyuridine (RP101), Cancer Res 63: pp5,745-5,753, 2003
- Mickley LA, Spengler BA, Knutsen TA, Biedler JL and Fojo T, Gene rearrangement: a novel mechanism for MDR-1 gene activation, J Clin Invest 99: pp1,947-1,957, 1997
- Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C and Darnell JE, Jr STAT3 as an oncogene, Cell 98: pp295-303, 1999
- Miyashita H, Takebayashi Y, Eliason JF, Fujimori F, Nitta Y, Sato A, Morikawa H, Ohashi A, Motegi K, Fukumoto M, Mori S and Uchida T, Uridine phosphorylase is a potential prognostic factor in patients with oral squamous cell carcinoma, Cancer 94: pp2,959-2,966, 2002
- Fan Z, Beresford PJ, Zhang D, Xu Z, Novina CD, Yoshida A Pommier Y and Lieberman J, Cleaving the oxidative repair protein Ape1 enhances cell death mediated by granzyme, A Nat Immunol 4: pp145-153, 2003
- Wakusawa S, Nakamura S and Miyamoto K, Establishment by adriamycin exposure of multidrug-resistant rat ascites hepatoma AH130 cells showing low DT-diaphorase activity and high cross resistance to mitomycins, Jpn J Cancer Res 88: pp88-96, 1997