| Combating Chemoresistance |

|

|

|
Page 4 of 4
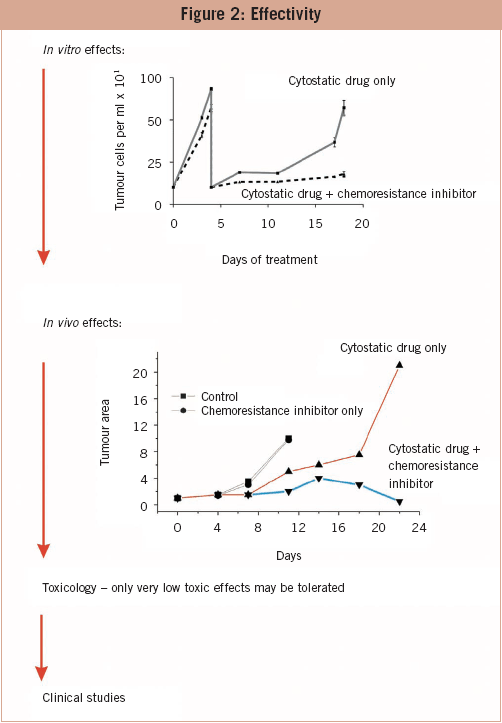
The effectivity of drugs on the molecular level is one matter; their effectiveness in vitro and in vivo another. In the long run, in vitro treatment of tumour cells with cytotoxic drugs leads to the induction of resistance and the increase of cells. Co-treatment with a chemoresistance inhibitor maintains the chemosensitivity of cells. Drugs that are not effective with respect to maintenance of chemosensitivity are excluded from further studies. On the next level, the candidates are tested in in vivo tumour models. Naked mouse experiments are not advisable because the prediction of effectiveness is not better than that of in vitro experiments. Candidates who pass this barrier are tested for their toxicological profile. Cytotoxic substances are most probably detected before the in vitro and in vivo experiments and have been already excluded. Only promising candidates reach the toxicology level. The most promising candidate is tested in clinical trials. To my knowledge, none of the drugs that are in the developmental pipelines of pharmaceutical and biotech companies throughout the world are intended to inhibit or prevent induction of chemoresistance. Drugs which circumvent or attack already existing chemoresistance address only gp-120 (MDR-1) with varying specificity, but gp-120 is only one of several reasons for chemoresistance. Inhibitors of receptors for the tyrosine kinase pathway such as ERBB2 (Herceptin) and inhibitors of the expression of single oncogens or STAT3 are in development too. However, the use of such drugs is restricted to tumours with a specific genetic profile.
References
|
||||||