|
Written by Biocom AG
|
|
Monday, 26 May 2008 |
Laborwelt Nr. 3 / 2008 - Verstärkung der Chemosensivität von Tumorzellen
Blitzlicht Krebs
Prof. Dr. Rudolf Fahrig, RESprotect GmbH, Dresden
Die Chemotherapie ist derzeit die Standardtherapie für Krebserkrankungen. Die für die Therapie eingesetzten Zytostatika treiben Tumorzellen in den Selbstmord. Leider ist die erfolgreiche Behandlungsperiode mit den gegenwärtig auf dem Markt befindlichen Zytostatika eist nicht lang genug, um den Tumor gänzlich zu vernichten. Der Tumor entwickelt nämlich Abwehrmechanismen gegenüber der Behandlung; er wird resistent. Die von RESprotect entwickelten Arzneimittel verhindern diese Resistenzbildung von Tumorzellen gegenüber Chemotherapien und erhöhen die Immunabwehr der Patienten. Im Gegensatz zu den seit Jahrzehnten bekannten und meist erfolglosen Versuchen, existierende Chemoresistenzen zu umgehen oder zu mindern, gibt es für diesen technologischen Ansatz weltweit keine Konkurrenz. Das erste von uns entwickelte Arzneimittel RP101 zeigte in Kombination mit Zytostatika in zwei kleinen klinischen Studien mit Bauchspeicheldrüsen-Krebspatienten eine lebensverlängernde Wirkung, welche jene bisheriger Therapien bei weitem übertraf. Darüber hinaus führte die RP101-Behandlung zu keinen schädlichen Nebenwirkungen, wie sie bei Behandlung mit Zytostatika immer zu beklagen sind. Da RP101 potentiell mit jedem Zytostatikum und bei allen Krebsarten wirken sollte, eröffnen sich neue Möglichkeiten für die Chemotherapie und damit für die Patienten.
|
|
Read more...
|
|
|
Written by Biocom AG
|
|
Monday, 19 May 2008 |
10th Guide to German Biotech Companies: German Biotechs 2008

|
RESprotect
Name
Address/P.O. Box
Postal Code/City
State
Contact Person
Telephone
Fax
Email Address
Internet Website
Number of Employees
Founded (year)
Type of Laboratory
Areas of Activity
External Collaborations
Request for
Further Collaborations
|
RESprotect GmbH
Fiedlerstr. 34
D-01307 Dresden
Saxony
Prof. Dr. Rudolf Fahrig
+49-351-4503200
+49-351-4503210
This e-mail address is being protected from spam bots, you need JavaScript enabled to view it
www.resprotect.com
10
2000
S1
Cancer chemotherapy;
Chemotherapy of infectious diseases
WITEGA/Berlin; Nycomed/Linz,
Austria; ERCOM/Budapest,
Hungary; Clinics Chemnitz;
University Leipzig; University
Munich; Technical University
Munich;University Vienna/
Austria; Avantogen/San Diego,
USA.
Partnering: RESprotect is looking for the appropriate partner to develop its key project RP101 in Europe, South America and Asia,
and its follow-on compounds worlwide.
|
- RESprotect - Prevention of Chemoresistance - Overview
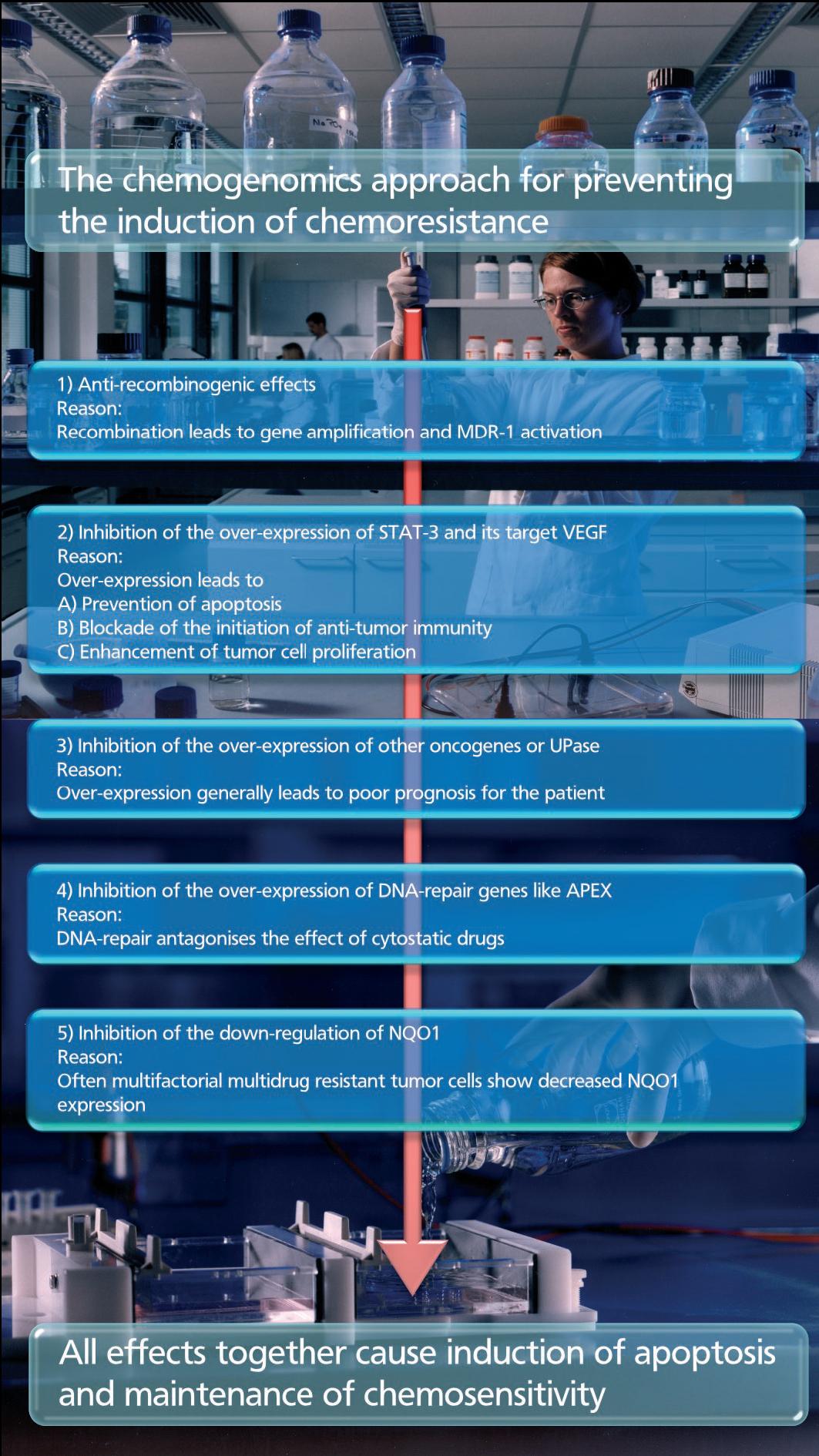
RESprotect GmbH is a privately owned biotechnology company located in Dresden Germany. RESprotect is focusing on the inhibition of chemoresistance and the enhancement of chemosensitivity. In contrast to the well known efforts to circumvent or decrease existing chemoresistance, this basic approach is unrivalled. RESprotect was founded in 2000. The founder is geneticist and came from the Fraunhofer-Institute for Toxicology in Hannover. At present clinical studies on RP101 are performed with pancreas cancer patients. The next generation of more efficient compounds is being explored, and a general broadening of the clinical indications is in process. RESprotect is in the position to enter a segment of the huge market of anticancer cytostatics. Use patents exist and an extension of the patent portfolio by substance patents was achieved in 2006. New chemical entities have been identified and introduced to the development pipeline.
- Combating Chemoresistance - Chemogenomics joins the battleground
In cancer model systems, chemoresistance is often mediated by a single gene, and, therefore, may in theory be inhibited by any drug that targets the product of that targets that gene. These drugs possess potency and specificity for only one of the several reasons for chemoresistance. For this reason, the chemogenomics approach focuses on small molecules, causing favorable phenotypic changes, and inhibiting or preventing the induction of chemoresistance. The drugs have to counteract the over-expression of apoptosis-antagonizing genes and to enhance the immune responses. By influencing not only one but a number of different validated targets a new class of effective anti-cancer drugs will be developed. These compounds have to be administered in addition to standard chemotherapy. RP101 is the first drug that shows these effects in tumor cells in cultured tumor cells, in animals and in patients.
- RP101 improves the efficasy of chemotherapy treatment in pancreatic carcinoma cells and patients
In pre-clinical studies, RP101 has shown strong antitumor effects due to inhibition of chemoresistance and the enforcement of apoptotic response upon drug treatment. RP101
|
|
affects numerous gene products related to chemoresistance and tumor immunity. In a Phase I study including five different tumor entities and 12 different cytostatic drugs, no enhancement of unwanted side effects had been observed. In a Phase II pilot study with 13 pancreas cancer patients, RP101 co-treatment enhanced remissions, survival and time to progression. The results of the pilot study were confirmed in a second study with 21 patients in similar stages of disease. The results were similar (Fahrig, et al., Anti-Cancer Drugs 17, 2006, 1045-56). Our two studies roughly showed the tendency of doubling the survival time. After one year, 4 to 5 times more patients with far metastases lived in the RP101 co-treatment group than in the chemotherapy alone group. In both studies, adverse events were consistent with those observed with the cytostatic drugs alone, or the underlying disease. The efficacy of RP101 exceeds all other regimens known to us. The data implicated that acquisition of chemoresistance was prevented and the antitumor efficacy of standard chemotherapy was improved. A double blind phase II/III study with 153 patients started in Q4 2007. 55 sites in Europe and America will participate in the study.
CEO – Founder:
Prof. Dr. Rudolf Fahrig
CSO – Cell and molecular biology:
Dr. Jörg-Christian Heinrich
CSO – Pharmacy and chemistry:
Dr. Dieter Lohmann
COO/CFO - Marketing and Organisation:
Dr. Torsten Fahrig
Finances: BW Kerstin Jahn
Nearly 7 million euros were raised by the company in the first round of financing. The next round was replaced by out-licensing the North-America rights of RP101. RESprotect has signed in September 2004 a license agreement with Australian Cancer Technology. The Australian Biotech company acquired the license for the use of the anti-cancer drug RP101 in North America. SciClone Pharmaceuticals Inc./ San Francisco aquired this lisence in 2007. SciClone finances the clinical development of RP101 in Europe and North America. RESprotect has free access to the data for approval of RP101 outside the USA and Canada.
|

RESprotect
|
|
|
|
Written by RESprotect GmbH
|
|
Wednesday, 06 February 2008 |
INITIATION OF A PHASE 2 TRIAL OF RP101 IN LATE-STAGE PANCREATIC CANCER PATIENTS
 Dresden, February 6, 2008: Dresden, February 6, 2008: RESprotect´s US development partner, SciClone Pharmaceuticals, Inc. (NASDAQ: SCLN) announced that the first patient has been dosed in its phase 2 clinical trial using RP101, a nucleoside analog which may act to enhance the beneficial effect of chemotherapy, for the treatment of pancreatic cancer. RESprotect, the inventor of the RP101 oncology technology, has granted in 2007 the exclusive rights in the United States and Canada to develop and commercialize RP101, a clinical-stage compound for the treatment of cancer, to SciClone Pharmaceuticals, Inc. (Nasdaq:SCLN). SciClone acquired the rights from Resistys, Inc., which acquired it 2004 from RESprotect. SciClone performs clinical studies with RP101. The results are freely available to RESprotect.
|
|
Read more...
|
|
|
Written by RESprotect GmbH
|
|
Thursday, 01 November 2007 |
RP101 Granted U.S. Orphan Drug Designation for Pancreatic Cancer
In November 2007, RP101 was granted Orphan Drug designation for the adjunct
treatment of pancreatic cancer by the U.S. Food and Drug Administration (FDA). This designation will allow
SciClone a seven-year period of market exclusivity once RP101 is approved. The Orphan Drug designation is intended to provide incentives to drug and biologics suppliers to develop and supply drugs for the treatment of rare diseases, currently defined as diseases that affect fewer than 200,000 individuals in the United States.
RESprotect will apply for the European EMEA orphan drug designation in 2009.
About RP101: RP101’s potential efficacy for treating cancer patients was discovered by the founder of RESprotect, Prof. Fahrig. RP101 has been evaluated in combination with cytostatic agents such as gemcitabine which is used to treat pancreatic, lung, ovarian and breast cancer patients. Although approved in several European countries for antiviral indications, RP101’s potential efficacy to combat chemoresistance and improve chemosensitivity constitutes a new clinical use for RP101 which is protected by three use patents by RESprotect. In two separate, unrelated phase 1 clinical trials with late-stage pancreatic cancer patients, RP101 was used in combination with gemcitabine, the current standard of care, or gemcitabine + cisplatin. The results were published in 2006 (Fahrig, et al., Anti-Cancer Drugs 17, 2006, 1045-56). In summary, RP101 co-treatment approximately doubled median survival. Moreover, 4 - 5 times more patients with far metastases (stage IV) survived one year or longer in comparison with the gemcitabine-alone group.
About RESprotect: RESprotect GmbH is a privately owned Biotech Company located in Dresden/Germany. RESprotect is focusing on the inhibition of chemoresistance and the enhancement of chemosensitivity. Chemogenomics, the approach of Resprotect, focuses on the application of small synthetic molecules, which elicit favourable phenotypic changes. The combination with genomic tools concentrating on specific biological pathways allows a better understanding of the broader effect of a drug. Thus, it is possible to discover drugs that target the cause of a disease rather than its symptoms. RESprotect's compounds are given additionally to standard chemotherapy. In contrast to the well known efforts to circumvent or decrease existing chemoresistance, RESprotect´s approach is unique.
|
|
|
Written by futureSAX GmbH Dresden
|
|
Saturday, 18 August 2007 |
|
5 Jahre futureSAX: Auszeichnung der Sieger
 18. August 2007. Der Sieger in der Kategorie "Wachsen" ist die RESprotect GmbH, Dresden (15.000 € Preisgeld) – Bio- /
Nanotechnologie: Den Hochschulsonderpreis teilen sich in diesem Jahr die
Hochschulnetzwerke SAXEED Chemnitz und SMILE Leipzig. Die durch die
Netzwerke betreuten Teams reichten nicht nur die meisten Konzepte ein,
sie konnten auch qualitativ überzeugen. 18. August 2007. Der Sieger in der Kategorie "Wachsen" ist die RESprotect GmbH, Dresden (15.000 € Preisgeld) – Bio- /
Nanotechnologie: Den Hochschulsonderpreis teilen sich in diesem Jahr die
Hochschulnetzwerke SAXEED Chemnitz und SMILE Leipzig. Die durch die
Netzwerke betreuten Teams reichten nicht nur die meisten Konzepte ein,
sie konnten auch qualitativ überzeugen.
In den neuen Räumen der Deutschen Werkstätten Hellerau GmbH werden heute
die Sieger des Businessplan-Wettbewerbs futureSAX2007 mit Unterstützung
der Ostsächsischen Sparkasse Dresden prämiert.
Der Schirmherr des
futureSAX, der Sächsische Staatsminister für Wirtschaft und Arbeit,
Thomas Jurk (SPD), wird die Preisträger in den Kategorien "Gründen" und
"Wachsen" auszeichnen. „In den vergangenen fünf Jahren wurden mit Hilfe
von futureSAX 129 innovative Unternehmen gegründet und über 1000
Arbeitplätze geschaffen", so Minister Jurk. "Die realisierten
Geschäftskonzepte spiegeln das breite technologische Spektrum wider,
für das Sachsen steht. Das zeigt, dass es 2002 der richtige Schritt
war, den IT-futureSAX zu einem branchenoffenen Wettbewerb zu
erweitern."
Den Hochschulsonderpreis für die aktivste Hochschule bzw.
Forschungseinrichtung überreicht Dr. Knut Nevermann, Staatssekretär im
Sächsischen Staatsministerium für Wissenschaft und Kunst. „Dieser
Wettbewerb zeigt, dass es an den Hochschulen in Sachsen ein enormes
Potenzial an Geschäftsideen gibt“, sagte Staatssekretär Dr. Nevermann.
Sachsens Hochschulen hätten deshalb die Aufgabe, Unternehmer- und
Forschergeist gezielt zu fördern und in Wissens- und
Technologietransferstrukturen einzubinden. „Es ist wünschenswert, dass
das Thema „Existenzgründungsmanagement“ an den Hochschulen ausgebaut
wird, damit Studierenden geholfen wird, persönliches Potenzial und
individuelle Stärken zu entdecken.“ Hochschulgründernetzwerke seien ein
ideales Sprungbrett für Studierende, Hochschulmitarbeiter und
Absolventen in die Unternehmen und das Unternehmertum, so
Staatssekretär Dr. Nevermann.
In der Kategorie "Gründen" haben folgende Teams die Jury überzeugen
können:
-
Preis: Team Phacon, Leipzig (15.000 € Preisgeld) –
Technologie
-
Preis: Team Urotec, Dresden (10.000 € Preisgeld) – Bio-/
Nanotechnologie
-
Preis: Team Menippos, Chemnitz (5.000 € Preisgeld) –
Informations- und Kommunikationstechnologie
Zum zweiten Mal wird der
Wachstumspreis an ein Unternehmen mit einem besonders innovativen
Erweiterungsvorhaben vergeben. Der Sieger in der Kategorie "Wachsen"
ist:
- die RESprotect GmbH, Dresden (15.000 € Preisgeld) –
Bio- / Nanotechnologie
Den Hochschulsonderpreis teilen sich in diesem
Jahr die Hochschulnetzwerke SAXEED Chemnitz und SMILE Leipzig. Die
durch die Netzwerke betreuten Teams reichten nicht nur die meisten
Konzepte ein, sie konnten auch qualitativ überzeugen.
Die Pressetexte der Preisträger finden Sie im
Anhang. O-Töne und weitere Informationen zum Wettbewerb stehen im
Presse-Downloadbereich unter www.futuresax.de zur Verfügung.
Ansprechpartnerin für die Presse: Beate Bartsch
Businessplan-Wettbewerb Sachsen GmbH
Pirnaische
Straße 9
01069 Dresden
Tel. 0351 4910-4095
Fax 0351 4910-4075
This e-mail address is being protected from spam bots, you need JavaScript enabled to view it
|
|
Read more...
|
|
|
Written by RESprotect GmbH
|
|
Monday, 07 May 2007 |
RESprotect grants U.S. and Canada License for its anti-cancer drug RP101 to SciClone Pharmaceuticals
Dresden, Germany – May 7, 2007 – RESprotect GmbH today announced that it has granted the exclusive rights in the United States and Canada to develop and commercialize RP101, a clinical-stage compound for the treatment of cancer, to SciClone Pharmaceuticals, Inc. (Nasdaq:SCLN). SciClone acquired the rights from Resistys, Inc., a wholly-owned subsidiary of Avantogen Oncology, Inc. (Nasdaq: AVTOE), which acquired the rights from RESprotect in 2004. As part of the transaction RESprotect GmbH will receive about 4.5% of common stock in Avantogen Oncology, Inc.07. Mai 2007.
Under the terms of the agreement, SciClone will pay approximately $1.7 million in upfront fees, a $1.3 million milestone payment upon initiation of a phase 2 clinical trial, post phase 3 success-based regulatory and commercial payments up to $22 million, and royalties on future sales to RESprotect GmbH and Avantogen Oncology, Inc. SciClone Pharmaceuticals, Inc. will fund all future clinical trial costs for U.S. and Canadian regulatory purposes necessary for the successful commercialization of RP101 program.
„We are delighted to sign this agreement with SciClone Pharmaceuticals, and look forward to working with SciClone in the continued development of RP101,“
said Prof. Rudolf Fahrig, Chief Executive Officer and founder of RESprotect GmbH.
Friedhelm Blobel, Ph.D., President and Chief Executive Officer of SciClone Pharmaceuticals, Inc. added “We are excited to complete this development and commercialization agreement. Our goal is to initiate a U.S. phase 2 study using RP101 to treat late-stage pancreatic cancer by the fourth quarter of 2007“.
|
|
Read more...
|
|
|