|
|
Written by LeadDiscovery Ltd.
|
|
Thursday, 12 October 2006 |
RP101 improves the efficacy of chemotherapy in pancreas carcinoma cell lines and pancreatic cancer patients.
(Source: Anticancer Drugs.) Full Citation:Anticancer Drugs. 2006 Oct;17(9):1045-1056.

Publisher's Abstract
Fahrig R, Quietzsch D, Heinrich JC, Heinemann V, Boeck S, Schmid RM, Praha C, Liebert A, Sonntag D, Krupitza G, Hanel M.
RESprotect, Dresden, Clinics for Internal Medicine II, Clinics for Internal Medicine III, Institute for Laboratory Medicine, Klinikum Chemnitz GmbH, Kuchwald, University Munich, Klinikum Grosshadern, Technical University Munich, Klinikum Rechts der Isar, Germany, Institute for Clinical Pathology, University Vienna, Vienna, Austria.
RP101 [(E)-5-(2-bromovinyl)-2'-deoxyuridine (BVDU)], which supports apoptosis and prevents the acquisition of chemoresistance, was tested in cultured human ancreatic tumor cells. RP101 downregulated uridine phosphorylase, a marker of poor prognosis, and APEX1, which is involved in DNA repair, and repressed Stat3 and its target vascular endothelial growth factor. Furthermore, RP101 activated antitumor immunity as demonstrated by enhanced cytolytic activity of NK-92 Natural killer cells. This was concomitant with an enhanced expression of lymphotoxins alpha and beta, natural killer cell transcript 4, tumor necrosis factor LIGHT/TNFSF-14, and intercellular adhesion molecule-1 in pancreas carcinoma cells. These results encouraged us to investigate the effect of RP101 in pancreas cancer patients. Here, we present data from two RP101 combination therapy schemes. In a first pilot study, 13 patients in stage III and VI of the disease were treated with gemcitabine +cisplatin+RP101. RP101 co-treatment enhanced remissions, survival and time to progression. Seventy-seven percent of the patients lived or have lived longer than 1 year, and 23% have lived more than 2 years. Median survival was 447 days, time to progression 280 days and the response rate 33%. A second study with 21 patients in similar stages of disease, treated with RP101+gemcitabine alone, confirmed the results of the pilot study. Eighty-three percent of the presently evaluable patients live or lived 0.5 years or longer and 33% 1 year or longer. Considering both studies, the tumor control was 94%. The data indicate that acquisition of chemoresistance was prevented and the antitumor efficacy of standard chemotherapy was improved. To our knowledge, RP101 co-treatment is more efficient than any other regimen published.
|
|
|
Written by BioCentury
|
|
Monday, 21 August 2006 |
Clinical Status 2006 -
Avantogen Oncology Inc. (AVTO), Los Angeles, Calif.
Bioaccelerate Holdings Inc. (BACL), New York, N.Y.
RESprotect GmbH, Dresden, Germany

-
Product: RP101
- Business: Cancer
- Molecular target: STAT3 mRNA; Apex gene
- Description: Inhibits induced chemoresistance and enhances chemosensitivity
- Indication: Treat advanced pancreatic cancer
- Endpoint: NA
- Status: Interim Phase I data
- Milestone: Submit IND (4Q06); start Phase II (early 2007)
- Interim data from an open-label Phase I trial in 22 patients showed that RP101 plus gemcitabine led to survival rates of 68% and 39% at 6 and 12 months, respectively. The estimated median survival was 9.3 months.
-
Product: RP101
- Business: Cancer
- Molecular target: STAT3 mRNA; Apex gene
- Description: Inhibits induced chemoresistance and enhances chemosensitivity
- Indication: Treat advanced pancreatic cancer
- Endpoint: NA
- Status: Phase II start
- Milestone: Start Phase II (early 2007)
- By early next year, AVTO will begin a Phase II trial comparing 750 mg daily of RP101 plus gemcitabine to gemcitabine plus placebo.
|
|
|
Written by Biocom AG
|
|
Sunday, 21 May 2006 |
8th Guide to German Biotech Companies 2006: German Biotechs 2006

|
RESprotect
Name
Address/P.O. Box
Postal Code/City
State
Contact Person
Telephone
Fax
Email Address
Internet Website
Number of Employees
Founded (year)
Type of Laboratory
Areas of Activity
Annual Turnover
Relevant R&D Budget
Biological Patents
External Collaborations
Request for
Further Collaborations
|
RESprotect GmbH
Fiedlerstr. 34
D-01307 Dresden
Saxony
Prof. Dr. Rudolf Fahrig
+49-351-4503200
+49-351-4503210
This e-mail address is being protected from spam bots, you need JavaScript enabled to view it
www.resprotect.com
10
2000
S1
Cancer chemotherapy;
Chemotherapy of infectious diseases
n.a.
n.a.
n.a.
WITEGA/Berlin; Nycomed/Linz,
Austria; ERCOM/Budapest,
Hungary; Clinics Chemnitz;
University Leipzig; University
Munich; Technical University
Munich;University Vienna/
Austria; Avantogen/San Diego,
USA.
Partnering: RESprotect is looking for the appropriate partner to develop its key project RP101 in Europe, South America and Asia,
and its follow-on compounds worlwide.
|
- RESprotect - Prevention of Chemoresistance - Overview
RESprotect GmbH is a privately owned biotechnology company located in Dresden Germany. RESprotect is focusing on the inhibition of chemoresistance and the enhancement of chemosensitivity. In contrast to the well known efforts to circumvent or decrease existing chemoresistance, this basic approach is unrivalled. RESprotect was founded in 2000. The founder is geneticist and came from the Fraunhofer-Institute for Toxicology in Hannover. At present clinical studies with RP101 in the pancreas cancer indication, exploratory research in the identification of next generation of New Chemical Entities (NCEs) and general broadening of clinical indications are underway. RESprotect is in a position to enter exclusively a segment in the huge market of anticancer cytostatics. Use patents exist and extension of the patent portfolio by substance patents is achieved. New chemical entities have been identified and introduced to the development pipeline.
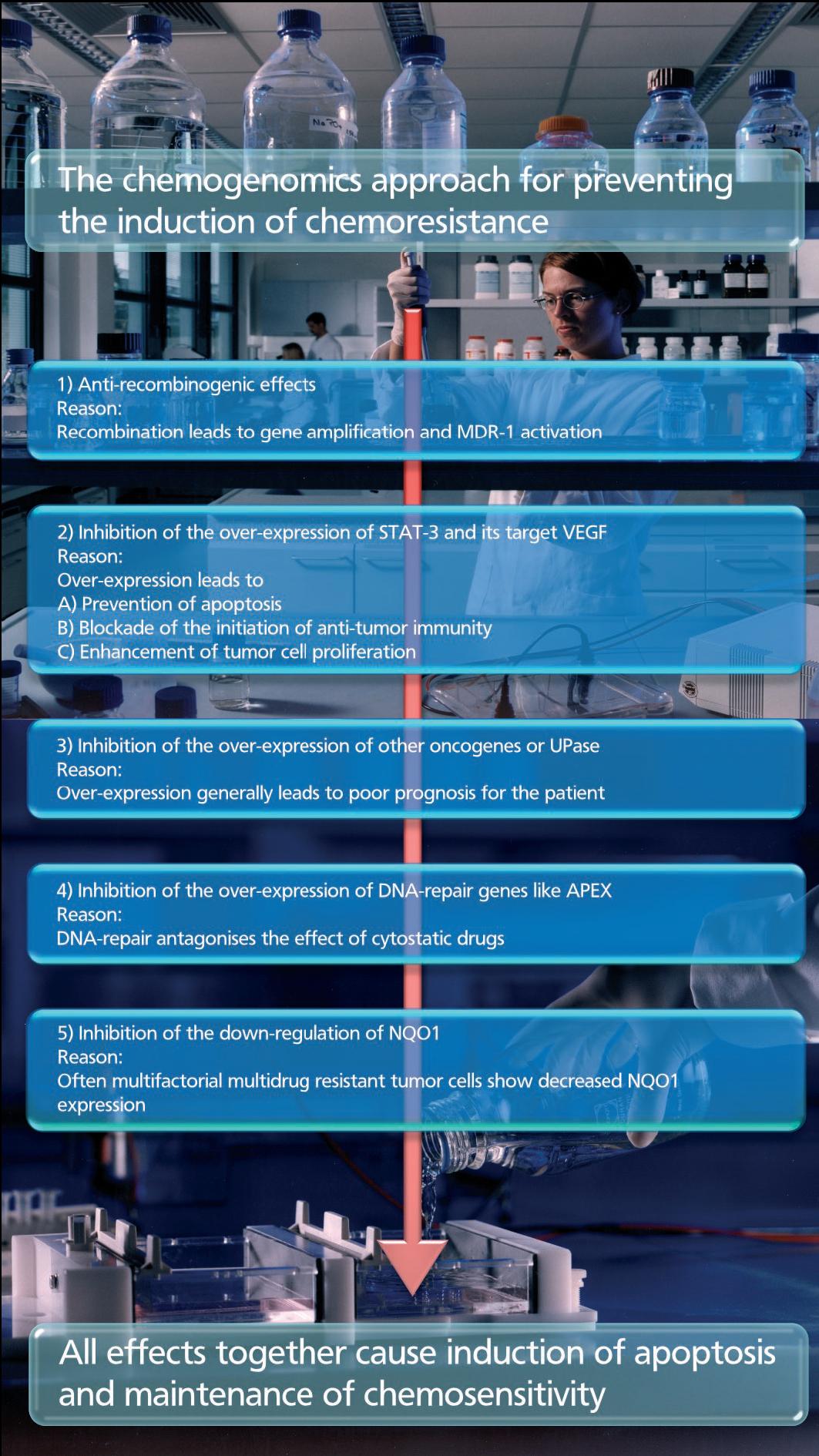
- Combating Chemoresistance - Chemogenomics Enters the Battleground
In cancer model systems, chemoresistance is often mediated by a single gene, and, therefore, may in theory be inhibited by any drug that targets the product of that gene. All these drugs possess potency and specificity exclusively for only one of the several reasons for chemoresistance. In this respect, the chemogenomics approach focuses on small molecules, causing favorable phenotypic changes, and inhibiting or preventing the induction of chemoresistance. The drugs have to counteract the over-expression of apoptosis-antagonizing genes and to enhance the immune responses. By influencing not only one but a number of different validated targets a new class of effective anti-cancer drugs will be developed. These compounds have to be administered in addition to standard chemotherapy. RP101 is the first drug that shows these effects in tumor cells in culture, in animals and in patients. RP101 improves the efficacy of chemotherapy in treating pancreatic carcinoma cells or patients.
In pre-clinical studies, RP101 has shown strong antitumor effects due to inhibition of
|
|
chemoresistance and the enforcement of apoptotic response upon cancer drug treatment. RP101 affects numerous gene products related to chemoresistance and tumor immunity. In a Phase I study including five different tumor entities and 12 different cytostatic drugs, no enhancement of unwanted side effects had been observed. In a Phase II pilot study with 13 pancreas cancer patients, RP101 co-treatment enhanced remissions, survival and time to progression. The results of the pilot study were confirmed in a second study with 21 patients in similar stages of disease. The results were not identical but similar. Our two studies roughly showed the tendency to double the survival time. In both studies, adverse events were consistent with those observed with the cytostatic drugs alone, or the underlying disease. The efficacy of RP101 exceeds all other regimens. The data implicated that acquisition of chemoresistance was prevented and the antitumor efficacy of standard chemotherapy was improved. A Phase II/III study with larger number of patients is in progress.
CEO – Founder:
Prof. Dr. Rudolf Fahrig
CSO – Cell and molecular biology:
Dr. Jörg-Christian Heinrich
CSO – Pharmacy and chemistry:
Dr. Dieter Lohmann
Finances:
Kerstin Jahn
Nearly 7 million euros were raised by the company in the first round of financing. The next round was replaced by out-licensing the North-America rights of RP101. RESprotect has signed in September 2004 a license agreement with Australian Cancer Technology. The Australian biotech company acquired the license for the use of the anti-cancer drug RP101 in North America. The drug is the first commercial breakthrough of RESprotect. AustCancer (new name Avantogen) will finance the clinical development of RP101 in Germany and the USA. RESprotect or its licensee will have free access to the data for approval of RP101 in Europe or elsewhere outside the USA and Canada.
|

RESprotect
|
|
|
|
Written by Samedan Ltd.
|
|
Tuesday, 02 May 2006 |
Combating Chemoresistance –
Chemogenomics Joins the Battleground

Drugs that prevent or inhibit induction of chemoresistance are beset
by limitations. Rudolf Fahrig at RESprotect GmbH investigates a new approach
Rudolf Fahrig is Professor at the University of Hamburg, Germany, and CEO of RESprotect GmbH. He served for many years in several national and international scientific committees and on the editorial boards of scientific journals. His work, resulting in a drug that prevents induction of chemoresistance, received the ‘Central German Innovation Prize’ in 2005. Rudolf studied Biology at the University of Hamburg, the Free University, Berlin, the University of Vienna, Austria and the Justus-Liebig-University.
In cancer model systems, chemoresistance is often mediated by a single gene, and therefore may in theory be inhibited by any drug that targets the product of that gene. All these drugs possess potency and specificity for only one of the several reasons for chemoresistance. In this respect, the chemogenomics approach focuses on small molecules, causing favourable phenotypic changes, and inhibiting or preventing the induction of chemoresistance. The drugs have to counteract the over-expression of apoptosis-antagonising genes and to enhance immune responses. By influencing not only one but a number of different validated targets, a new class of effective anticancer drugs will be developed. These compounds have to be given in addition to standard chemotherapy. RP101 (BVDU) is the first drug that shows these effects in vitro and significantly extended patient survival rate in a clinical pilot study. Similar tendencies are observed in a second study that has started recently.
|
|
Read more...
|
|
|
Written by Capital of Saxony Dresden
|
|
Monday, 20 March 2006 |
Living science - Biotechnology in Dresden

Prof. Dr. Rudolf Fahrig
Managing Director, RESprotect GmbH
- The environment is perfect
There are at least three good reasons for innovative biotechnology companies to settle in Dresden. Effective networks such as BioMeT Dresden and close proximity to clinics and research facilities like the Ma Planck Institute are equally important site factors as is the open-mindedness of the population towards biotechnology. Finally, with is wide range of cultural offerings, Dresden is one of the most beautiful major cities in Germany. In short: It simply is very attractive to live and work in Dresden.
|
|
|
Written by BioMeT e.V. Dresden
|
|
Wednesday, 15 March 2006 |
BioMet Mail 1|2006

42 Vereins- und Netzwerkmitglieder waren neugierig und fanden am 23. Januar trotz Eiseskälte den Weg nach Hellerau.
Nach der Begrüßung wurden zunächst die schon etwas länger in Dresden ansässigen Unternehmen RESprotect GmbH und Qualitype AG und dann die 2005 angesiedelte InnoTERE BioMaterials GmbH vorgestellt.
Herr Prof. Dr. Rudolf Fahrig, Geschäftsführer der RESprotect GmbH, stellte die Entwicklung seines Unternehmens seit der Gründung im Jahr 2000 und seine international verknüpften Forschungsarbeiten zur Verhinderung von Chemoresistenz am Beispiel des Bauchspeicheldrüsenkrebses dar. Die RESprotect GmbH erhielt für ihre herausragenden Leistungen als einer von 5 Preisträgern den Innovationspreis Mitteldeutschland 2005.
Die Qualitype AG, ein florierendes Bioinformatik-Unternehmen, gegründet 2001 in Hellerau, wurde von Herrn Dr. Frank Götz vorgestellt. Das Unternehmen bietet informationstechnische Leistungen für die Bereiche der biologischen, pharmazeutischen und medizinischen Forschung an. Zu den Erfolgen des Unternehmens zählt Qualiproof , eine Datenbank zum Salmonellenmonitoring in der Schweine-fleischerzeugung, die von der Qualitype AG entwickelt wurde und gepflegt wird. Daran beteiligen sich mehr als 25.000 Unternehmen mit über 100.000 Datensätzen. Als weiteres Geschäftsfeld entwickelt Qualitype AG Lösungen im Bereich der elektronischen Datenerfassung (EDC) für human- und veterinärmedizinische Studien. Außerdem werden die Unternehmen zu Fragen der Datenverwaltung, der Prozessanalyse, der Workflow-Optimierung oder der Validierung beraten.
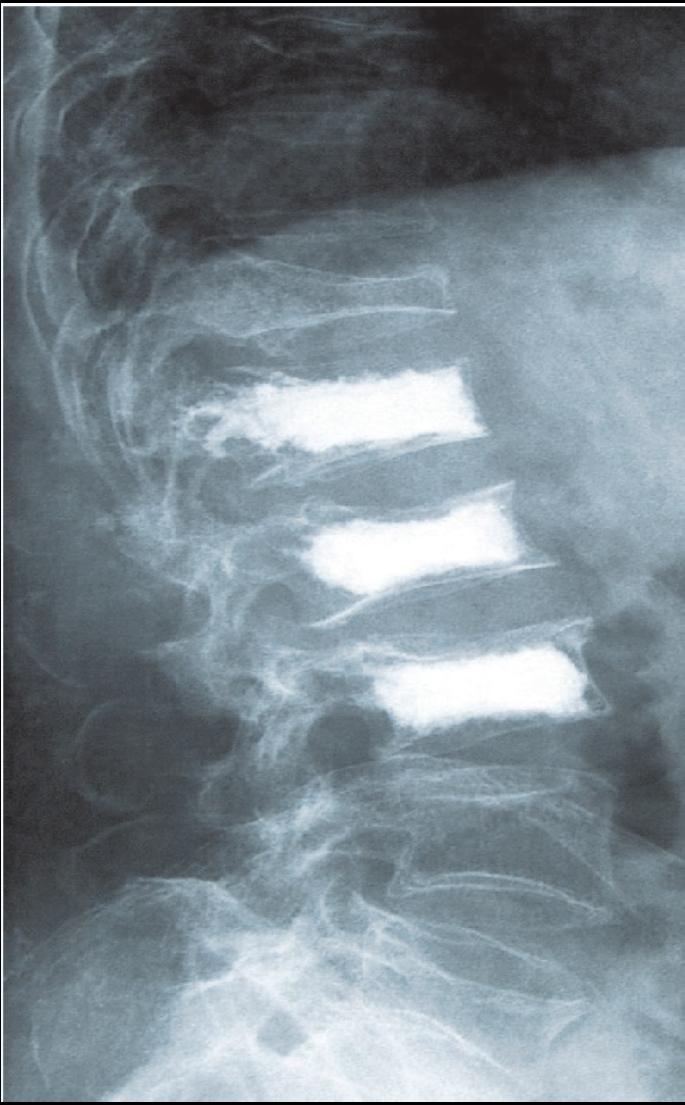
Eines der Geschäftsfelder der InnoTERE GmbH wird die Herstellung von bioaktivem Knochenzement zur Behandlung und Aussteifung ostheoporotischer Knochen sein.
Hier am Beispiel der Wirbelsäule.Die InnoTERE BioMaterials GmbH - Innovation für Tissue Engineering und Regeneration - stellte deren Geschäftsführer, Herr Dr. Berthold Nies, vor. Das Unternehmen wurde 2004 gegründet und ist seit Herbst im BioZ Dresden ansässig. Der Standort Dresden wurde wegen der Nähe zu den wichtigsten wissenschaftlichen Partnern, wie dem Max-Bergmann- Zentrum, gewählt. In einem gut illustrierten Vortrag zeigte Dr. Nies, welche Forschungsleistungen auf dem Gebiet der regenerativen Medizin zur Knochenregeneration, für das Verankern von Knochenimplantaten und zur Behandlung von Knochenschäden durch sein Unternehmen erbracht wurden und werden. Die InnoTERE GmbH ist einer der 10 Sieger des vom BMBF ausgeschriebenen Wettbewerbs für Medizintechnik 2005. Nach ihren Vorträgen beantworteten die Referenten zahlreiche Fragen aus dem Auditorium.
Wir bedanken uns ganz herzlich bei den drei Referenten, die uns auch die Abbildungen auf dieser Seite zur Verfügung stellten.

Im Labor der RESprotect GmbH. Vorn links Herr Professor Fahrig, Geschäftsführer des Unternehmens.
|
|
|
Written by Southern Cross Equities Ltd.
|
|
Friday, 10 February 2006 |
|

If there's one thing guaranteed to excite attention in the biotech investment field, it's the result of a clinical trial. This analyst could talk until he was blue in the face about in vitro data, findings from animal model work, and even anecdotal evidence that a therapeutic approach works in people. However until a company is ready to report the outcome of a properly designed clinical study, only the aficionados will really be listening, and they generally aren't people who buy much biotech stock. That's why a company called Avantogen, ASX Code ACU, is of some interest to this analyst at the moment.
If the name Avantogen doesn't ring a bell, you may know it by its pre-May 2005 name of Australian Cancer Technology, which also had the ACU ticker. When this analyst first looked at AustCancer around three years ago it was a more or less obscure developer of peptide-based cancer vaccines. Things began to change for the company in 2003 when Paul Hopper, a former private hospital executive turned technology entrepreneur, introduced a second project and helped recapitalise the company before being named CEO in August 2003, at the height of that year's biotech bull market.
Hopper had no background in biotech but he took seriously the task of going up the learning curve, and was able to do so fairly swiftly. He was also a good dealmaker, and over the next year and a half managed to bring into AustCancer a number of projects which both spread the project risk and moved the company closer to a point where marketable products would be in sight. For a while in 2003 and 2004 the market was pretty impressed. At its high point in May 2004 AustCancer stock was up fivefold on its level of twelve months before.
One of the projects that Hopper and his colleagues got hold of in 2004, and the chief reason to be looking at Avantogen right now, involves a small molecule drug that has been given the codename RP101. The compound has been showing promise as the next big thing in treating pancreatic cancer, and a Phase I/IIa clinical trial is currently being completed, with results expected in the next few months.
Sure, cancer of the pancreas isn't all that common in the Western world - there are only around 30,000 cases a year in the US - but uncommon medical conditions are often the most lucrative to target. A drug company can and often does charge the earth for drugs specific to a rare disease, and in the case of pancreatic cancer Avantogen has previously speculated that the U.S. market for RP101 is worth perhaps US$200m a year. Moreover if there's nothing out there that really works for the patients - and that seems to be the case with pancreatic cancer - the regulatory folks will generally accelerate approval of the drug.
And don't forget the tax breaks an other benefits out there for people who develop so-called 'Orphan Drugs' where the patient population is less that 200,000. All of this suggests that the pay-off from a successful clinical trial of RP101 could be both swift and lucrative from the perspective of a small company like Avantogen. Given RP101's performance to date, we think it's reasonable to expect the data from the trial to be favourable. But to understand why it's necessary to go back and look at all that early stage science. So bear with us for a moment while we explain why Avantogen thinks it is on to something good...
|
|
Read more...
|
|
|
<< Start < Prev 1 2 3 4 5 6 7 8 9 Next > End >>
|
| Results 22 - 28 of 59 |